Meet Melinta
A diverse company of believers and visionaries. We are the company of the moment and the enterprise of the future. We can’t wait to make legendary strides. Every step counts.
Our Mission
To provide innovative therapies to people impacted by acute and life-threatening illnesses.
Our Vision
All people who need our therapies will receive them.
Looking to the future of Melinta, I’m hopeful because I’ve seen first-hand the passion that my colleagues share in making a difference for the patients and customers who are counting on us every day. With that continued passion, the sky is the limit for Melinta.
– Ryan Yost, Key Account Manager, Fairfax, VA


Essential Behaviors
Essential Behaviors are the fundamental, indispensable and reoccurring actions that drive cultural success

Do the Right Thing
Be unequivocally open, honest, trustworthy and ethical; say what you mean and mean what you say.

Own It, Do It, Love It
Act now. Be accountable and passionate in all that you do.

Let Me Walk in Your Shoes
With high emotional intelligence, we have empathy and understand and care for our people, our patients and our partners. Listen with respectful curiosity.

Act Bold, Be Different, Create Value
Be innovative and courageous. Solve the right problems and don’t be afraid to fall forward. Be proactive and speak up.

We’ve Got Your Back
Be a champion for our people, patients and products.

Understand Before Being Understood
Be inclusive and approachable as you collaborate with others.

Be the Fun
Bring infectious energy and a positive outlook to everything you do.
Code of Conduct
At Melinta, we are committed to ensuring that everything we do to achieve our mission is rooted in the highest standards of integrity and complies with the laws and regulations that govern our industry. It is the foundation upon which we operate every day– ensuring that our activities and interactions are always anchored in the principles of respect, trust, and integrity and are focused on the care of patients.
Leadership
Christine Ann Miller
President and Chief Executive Officer
Kristen Allgor
Chief Human Resources Officer
John Harlow
Chief Commercial Officer
Jennifer Sanfilippo
General Counsel and Chief Compliance Officer
Susan Blum
Chief Financial Officer
Kelley Ford, PhD
Vice President, Manufacturing and Supply
Technical Operations, Supply Chain, Quality
Doug Girgenti, M.D
Vice President of Drug Development
Jisoo Park
Chief Business Officer
Pete Piliero, M.D.
Vice President of Medical Affairs
Board of Directors
Christine Ann Miller
President & CEO
Melinta Therapeutics
Sumner Anderson
Partner, Deerfield Management Company
Jonathan Leff
Partner, Chairman of The Deerfield Institute
Bryan Sendrowski
Partner, Deerfield Management Company
Melinta Partners
Our commitment is to ensure that all people who need our therapies can get them. We partner with other companies that share our commitment so our therapies can reach patients worldwide.
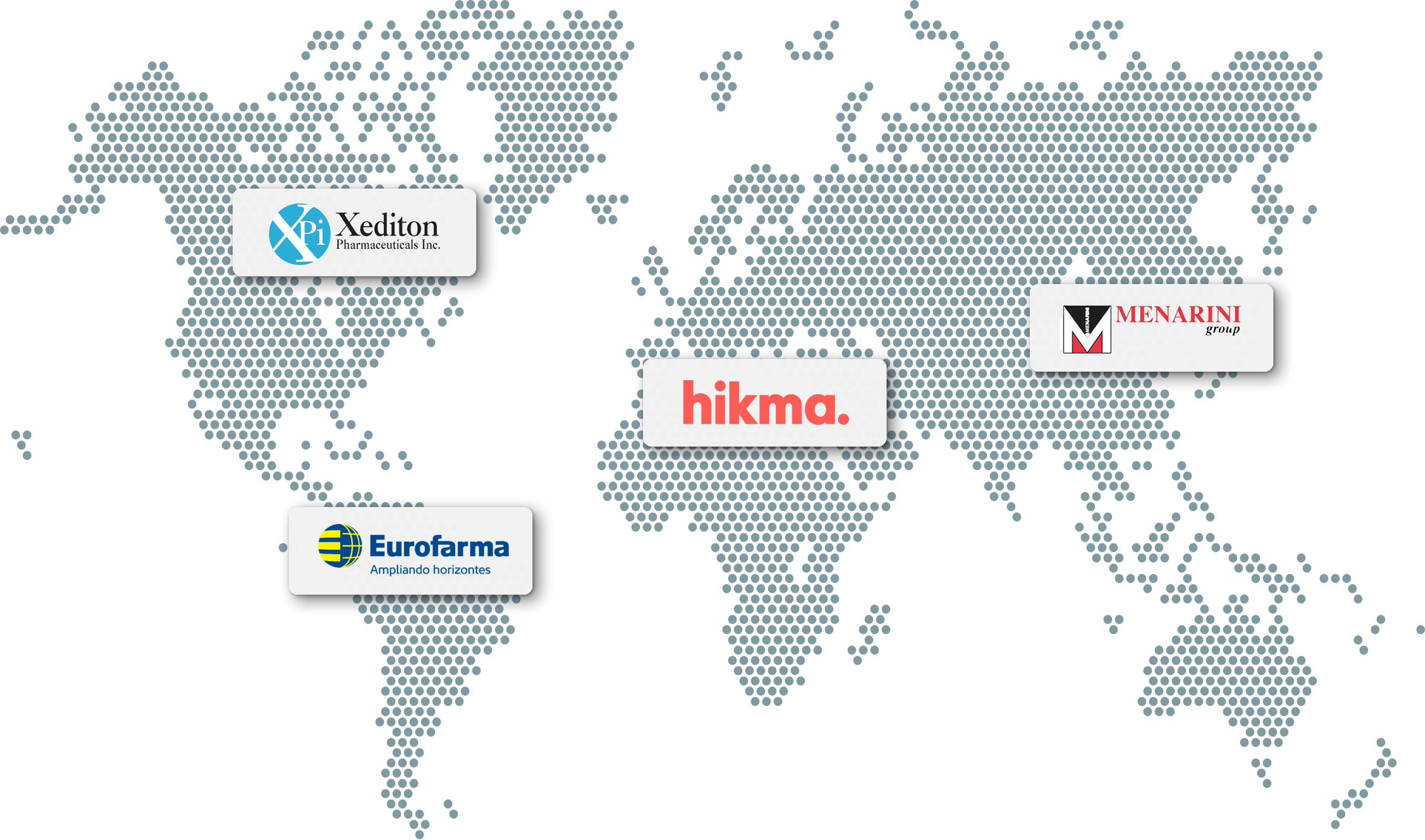
Hikma: delafloxacin, oritavancin, meropenem and vaborbactam
Menarini: delafloxacin, oritavancin, meropenem and vaborbactam
Xediton: delafloxacin, oritavancin, meropenem and vaborbactam
Smart and Choiceful
We ensure every choice we make has a business case that makes sense. Because our strong commercial engine is built for success.